Collaboration with Nordic Pharmaceutical Forum
Published:
Changes
To further strengthen the cooperation between the Nordic countries, FINOSE enters a collaboration with the New Expensive Drugs (NED) section of Nordic Pharmaceutical Forum.
The collaboration aims to support joint Nordic negotiations for products assessed through FINOSE, by highlighting points of contact and communication between NED and FINOSE. Joint HTA through FINOSE and joint negotiations with NED are conducted after agreement with the health technology developer.
The possibility of joint HTA and negotiations is offered as a route for suitable products and aims at equal patient access in the Nordic countries. The main steps of the process are outlined below.
Points of contact between FINOSE[1] and New Expensive Drugs (NED)[2] throughout the health technology assessment (HTA) and negotiation processes.
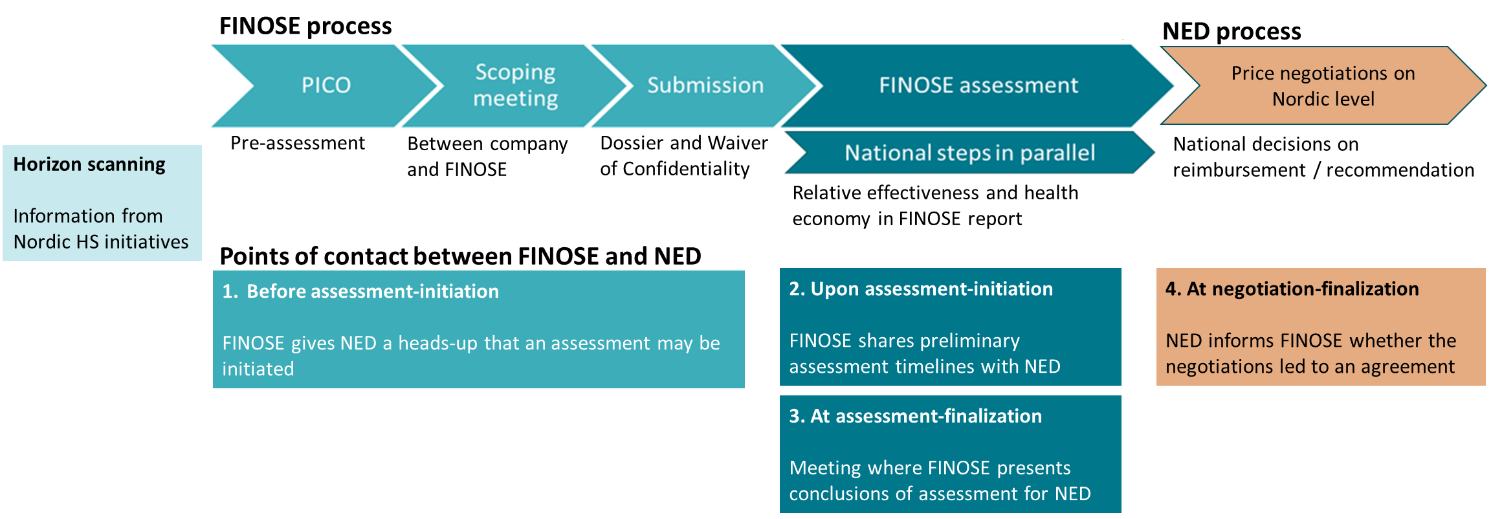
[1] FINOSE is a collaboration for joint Nordic HTA between the Danish Medicines Council (DMC), the Finnish Medicines Agency (Fimea), the Norwegian Medicines Agency (NoMA) and the Swedish Dental and Pharmaceutical Benefits Agency (TLV).
[2] NED is a working group in the Nordic Pharmaceutical Forum. The group consists of the price negotiation authorities, Amgros I/S in Denmark, Sykehusinnkjøp HF, divisjon legemidler (LIS) in Norway, Landspitali National University Hospital of Iceland, and the New Therapies council (NT-council) in Sweden.
Read more about Nordic Pharmaceutical Forum and their work with joint Nordic negotiations at Nordic Pharmaceutical Forum.