General obligations for manufacturers of medical devices
Published:
Changes
A manufacturer is a physical or legal person who manufactures or fully refurbishes a device or has a device designed, manufactured or fully refurbished, and markets that device under its name or trade mark.
What requirements apply to a manufacturer?
The table below lists some of the requirements, but the list is not exhaustive. The requirements that are relevant for each individual manufacturer also depend on the type of medical device and the device's risk classification. The requirements and obligations of manufacturers are found in several parts of MDR and IVDR and it is important that manufacturers familiarise themselves with the entire regulations.
| Article | Overview of some of the requirements |
| MDR Article 10 IVDR Article 10 |
General obligations for:
See separate page for special requirements for custom made devices.
|
| MDR Article 11 IVDR Article 11 |
Requirement for an authorised representative for manufactureres outside the EU/EEA or Turkey |
| MDR Article 15 IVDR Article 15 |
Requirement for a person responsible for regulatory compliance |
| MDR Article 18 | Requirements for patient information and implant cards for implantable medical devices |
| MDR Article 22 | Requirements for systems and procedure packs |
| MDR Article 29 IVDR Article 26 |
Requirements for registration of devices |
| MDR Article 31 IVDR Article 28 |
Requirements for registration of manufacturers and single registration number |
| MDR Article 32 IVDR Article 29 |
Requirements for summary of safety and clinical performance |
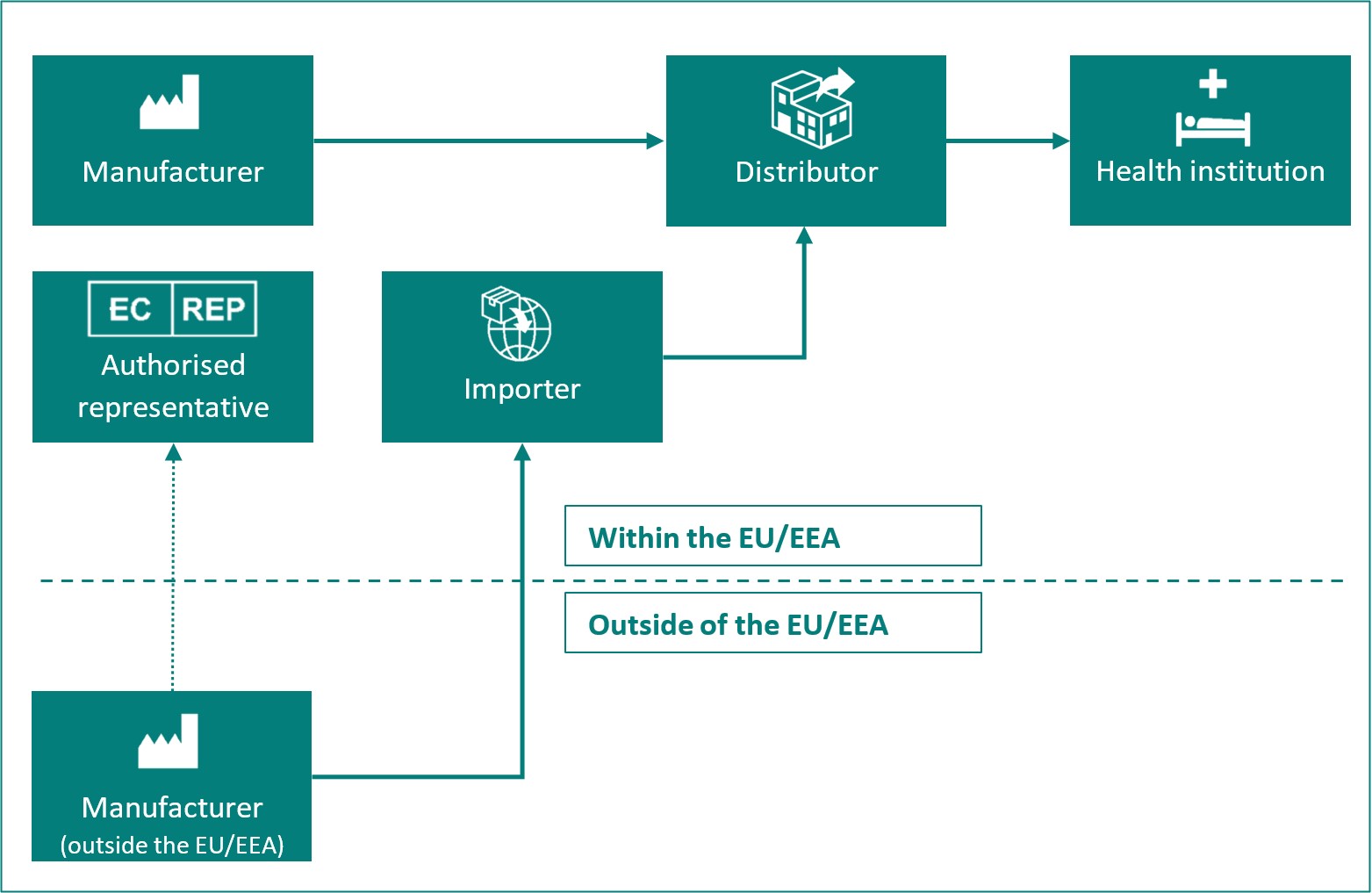
Overview of the different roles of economic operators:
| Economic operator |
Definition in MDR Article 2 and IVDR Article 2 |
| Manufacturer | A natural or legal person who manufactures or fully refurbishes a device or has a device designed, manufactured or fully refurbished, and markets that device under its name or trademark. |
| Authorised representative | Any natural or legal person established within the Union* who has received and accepted a written mandate from a manufacturer, located outside the Union*, to act on the manufacturer's behalf in relation to specified tasks with regard to the latter's obligations under this Regulation. |
| Importer | Any natural or legal person established within the Union* that places a device from a third country on the Union* market. |
| Distributor | Any natural or legal person in the supply chain, other than the manufacturer or the importer, that makes a device available on the market, up until the point of putting into service. |
| Health institution | An organisation the primary purpose of which is the care or treatment of patients or the promotion of public health. |
EU regulations on medical devices