Distance Sales/Online sales/E-commerce
Published:
Changes
Information on the requirements that apply to medical devices for online sales, free samples and when importing for personal use.
Page contents
Medical devices must fulfil the requirements of the regulation on medical devices if the following are offered:
-
electronic commerce or other information society services
-
to deliver a diagnostic or therapeutic service, for a fee or free of charge
Everyone who offers medical devices or services of this type must have a copy of the EU Declaration of Conformity for the device available.
If the devices offered via such services are not safe and secure, it may be required on grounds of protection of public health that the supplier ceases its activities, see MDR and IVDR article 6.
Requirements for free samples of medical devices
The essential requirements for free samples are the same as for other medical devices. This applies, among other things, to the requirements for CE marking, and Norwegian labelling and instructions for use.
Other relevant information regarding the distribution of free samples of medical devices:
- Regulations on restrictions on healthcare professionals' access to receive a gift, commission, service or other benefit (in norwegian).
Import of medical devices for personal use - via distance sale or physically
For everyone who purchases medical devices for personal use, it is important to note the requirements for those who sell medical devices via distance selling in Norway. If you buy a medical device for personal use via distance selling, you should make sure that the device is CE marked and that it comes with Norwegian labelling and Norwegian instructions for use. Choose reputable retailers and pay attention to what you buy.
For private individuals who physically buy a medical device in a third country and bring it back to Norway, only for personal use, the device does not have to meet the requirements of the regulations for medical devices.
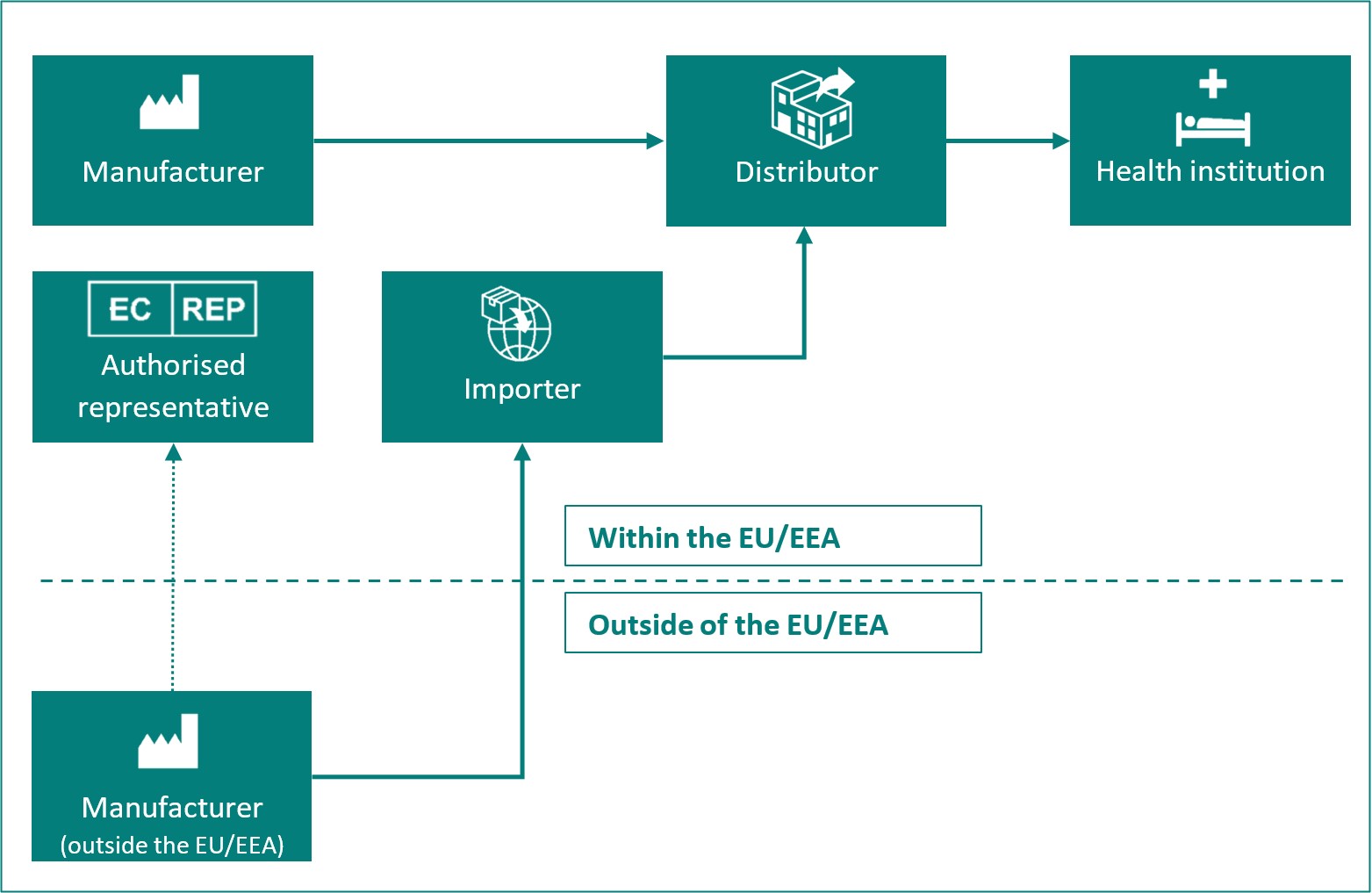
Overview of the different roles of economic operators:
| Economic operator |
Definition in MDR Article 2 and IVDR Article 2 |
| Manufacturer | A natural or legal person who manufactures or fully refurbishes a device or has a device designed, manufactured or fully refurbished, and markets that device under its name or trademark. |
| Authorised representative | Any natural or legal person established within the Union* who has received and accepted a written mandate from a manufacturer, located outside the Union*, to act on the manufacturer's behalf in relation to specified tasks with regard to the latter's obligations under this Regulation. |
| Importer | Any natural or legal person established within the Union* that places a device from a third country on the Union* market. |
| Distributor | Any natural or legal person in the supply chain, other than the manufacturer or the importer, that makes a device available on the market, up until the point of putting into service. |
| Health institution | An organisation the primary purpose of which is the care or treatment of patients or the promotion of public health. |